Free shipment over 70€ *** -10% on first cart with BeMore *** Free shipment over 70€ *** -10% on first cart with BeMore
How We Tested Bluegold Essence: Inside Our Clinical Study

Transparency Matters: Here’s Exactly How We Proved Our Claims
In the world of hair care, promises are everywhere. Clinical proof is rare. When we decided to submit Bluegold Essence RECONSTRUCTIVE HAIR TREATMENT to independent testing, we chose the rigorous path—not because it was easy, but because you deserve to know exactly what you’re putting on your scalp and exactly what it can do.
Here’s the complete story of how our clinical study was conducted.
The Testing Facility
We partnered with SGD Clinic (Specjalistyczny Gabinet Dermatologiczny Aplikacyjno-Badawczy) in Kraków, Poland—a specialized dermatological research facility. The study was supervised by two board-certified dermatologists and venereologists: Dr. Marek Brzewski, MD, PhD (registration 2396030) and Dr. Paweł Brzewski, MD, PhD (registration 2293825).
The sample was processed through Eurofins Polska, an accredited analytical laboratory, ensuring chain-of-custody documentation and microbiological purity verification before human testing began.
The Regulatory Framework
This wasn’t informal observation. The study followed strict protocols:
- EU Regulation 1223/2009 regarding cosmetic products
- EU Regulation 655/2013 regarding claims for cosmetic products
- Cosmetics Europe Guidelines for Product Testing and Assessment of Human Skin Compatibility (1997)
- Cosmetics Europe Guidelines for the Evaluation of the Efficacy of Cosmetic Products (2008)
- Helsinki Declaration principles for ethical human research
Every step was documented, every measurement recorded, every protocol followed.
The Participants
Ten female volunteers were selected based on strict inclusion and exclusion criteria:
Who could participate:
- Women over 18 years of age
- Healthy individuals
- Normal scalp skin without irritation or pathology
Who was excluded:
- Pregnant or lactating women
- Anyone with known allergies to product ingredients
- People with active alopecia areata or advanced androgenic alopecia
- Anyone who had recently undergone chemotherapy
- Those using treatments including minoxidil, corticosteroids, anti-inflammatory agents, or other hair-loss medications
The resulting group represented real diversity: ages ranged from 18 to 58, with various hair textures (fine to medium), different porosity levels, and both colour-treated and natural hair. Some had sensitive scalps; others dealt with mild seborrheic conditions. This wasn’t a cherry-picked group—it was a genuine cross-section of women seeking hair improvement.
The Protocol
Each participant received Bluegold Essence with clear instructions:
Application method: Spread the product evenly on the scalp and massage with fingertips or a massager Frequency: Once daily Duration: 12 weeks
Participants committed to using the product consistently and avoiding other hair treatments with similar claims during the study period. They remained in contact with supervising dermatologists throughout, with instructions to report any changes or concerns immediately.
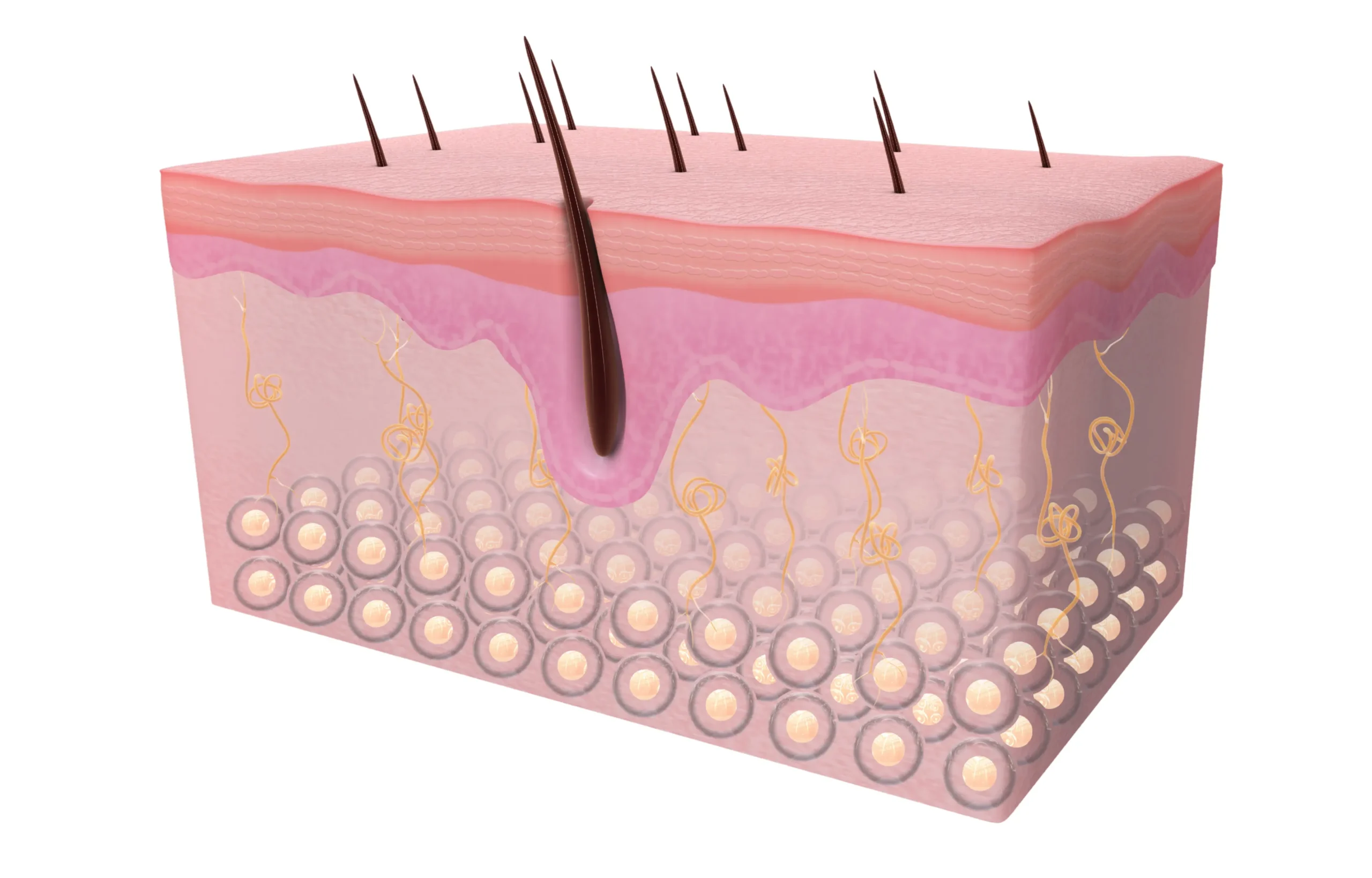
The Measurement Technology
Results were measured using the FotoFinder Trichoscale system—professional trichoscopy equipment used by dermatologists worldwide for precise hair and scalp analysis. This technology captures:
- Total hair count in a defined measurement area
- Hair density (hairs per square centimetre)
- Terminal vs. vellus hair ratio (thick, mature hairs vs. fine, thin hairs)
- Mean hair thickness in micrometres
- Cumulative hair thickness (total hair volume per area)
- Follicular unit analysis (how many hairs grow from each follicle)
Measurements were taken at baseline (before treatment began) and after 12 weeks of daily use, using identical measurement areas for accurate comparison.
The Safety Assessment
Beyond efficacy, dermatologists evaluated tolerance and safety. At study conclusion, all 10 participants reported:
- No scaling of the skin
- No itching
- No burning sensation
- No redness
- No feeling of skin tension
- No drying effects
- No signs of irritation or allergic sensitization
A perfect safety record across all participants.
The Results
The clinical conclusions, documented in Report No. A/371/01/2025:
- The product causes no side effects, irritation, or sensitization
- The product is well tolerated by the skin
- The product strengthens hair and scalp
- The product refreshes skin and helps reduce sebum production
- The product increases scalp hydration
- The product reduces skin redness
- The product increases growth of total hair count and average hairs per follicular unit
- The product increases terminal hair count, density, and thickness
Why This Matters
Anyone can make claims. Clinical documentation requires investment, time, and willingness to accept whatever results emerge. We submitted Bluegold Essence to independent scrutiny because we believed in what we’d created—and because you deserve evidence, not just promises.
This is the foundation of trust.